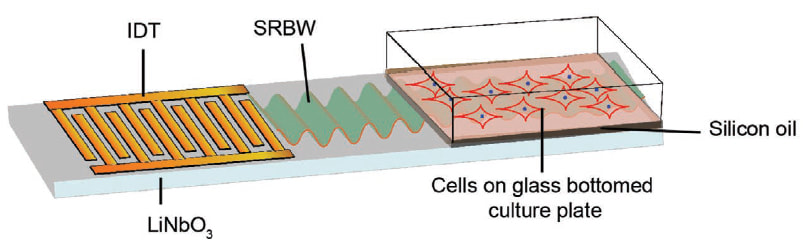
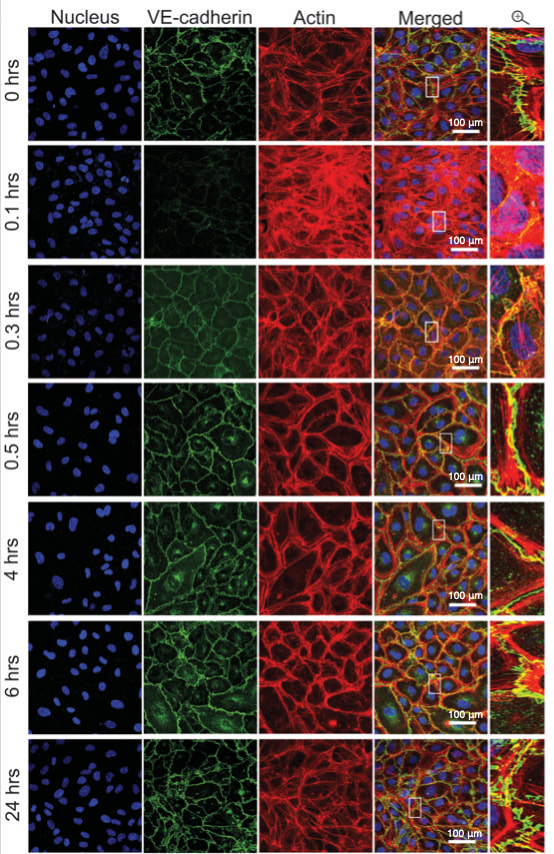
|
Cells have an innate ability to respond to a range of mechanical stimuli through their complex internal machinery. Such machinery consists of various mechanosensory elements that allows the cell to detect mechanical cues, and diverse cytoskeletal structures that transmit the force to different parts of the cell, where they are transcribed into complex transcriptomic and signalling events that determine their response and fate. In contrast to static mechanostimuli primarily involving constant-force loading such as compression, tension and shear (or forces applied at very low oscillatory frequencies (≤ 1 Hz) that essentially render their effects quasi-static), dynamic mechanostimuli comprising more complex vibrational forms (e.g., time-dependent, i.e., periodic, forcing) at higher frequencies are less well understood. Our work aims to elucidate the mechanotransductive processes associated with such high frequency acoustic forcing, especially that at MHz-order frequencies where cavitation is primarily non-existent (Rezk et al., J Phys Chem Lett 11, 4655, 2020). Historically, these frequencies have not been expected to yield appreciable cellular responses. On the contrary, we have discovered that cells do not just have the ability to sense external mechanostimuli at MHz-order frequencies through a combination of poration-free mechanisms such as mechanosensitive ion channels or transient membrane dilatory effects, but that they are also able to invoke considerably different second messenger (Ca2+) mobilisation dynamics that result in distinct downstream signalling cascades and hence cellular processes, with potential applications particularly for regenerative therapeutics (e.g., exosome biogenesis, stem cell differentiation, endothelial barrier modulation, etc.) (Ambattu & Yeo, Biophysics Rev 4, 021301 (2023)). |
Representative Publications
- H Li, J Friend, L Yeo, A Dasvarma, K Traianedes. Effect of Surface Acoustic Waves on the Viability, Proliferation and Differentiation of Primary Osteoblast-Like Cells. Biomicrofluidics 3, 034102 (2009).
- S Ramesan, AR Rezk, C Dekiwadia, C Cortez-Jugo, LY Yeo. Acoustically-Mediated Intracellular Delivery. Nanoscale 10, 13165–13178 (2018).
- S Ramesan, AR Rezk, LY Yeo. High Frequency Acoustic Permeabilisation of Drugs Through Tissue for Localised Mucosal Delivery. Lab Chip 18, 3272–3284 (2018) [Article coverart selected for journal back cover p 3324].
- LA Ambattu, S Ramesan, C Dekiwadia, E Hanssen, H Li, LY Yeo. High Frequency Acoustic Cell Stimulation Promotes Exosome Generation Regulated by a Calcium-Dependent Mechanism. Commun Biol 3, 553 (2020).
- S Ramesan, AR Rezk, PM Cevaal, C Cortez-Jugo, J Symons, LY Yeo. Acoustofection: High Frequency Vibrational Membrane Permeabilisation for Intracellular siRNA Delivery into Non-Adherent Cells. ACS Appl Bio Mater 4, 2781–2789 (2021).
- LA Ambattu, A Gelmi, LY Yeo. Short-Duration High Frequency MegaHertz-Order Nanomechanostimulation Drives Early and Persistent Osteogenic Differentiation in Mesenchymal Stem Cells. Small 18, 2106823 (2022) [Article coverart selected for journal back cover 18, 2270038].
- LA Ambattu, LY Yeo. Sonomechanobiology: Vibrational Stimulation of Cells and its Therapeutic Implications. Biophysics Rev 4, 021301 (2023) [Article selected for journal front cover].
- LA Ambattu, C Knight, K-h Lin, A Gelmi, LY Yeo. Calcium-Dependent cAMP Mediates the Mechanoresponsive Behaviour of Endothelial Cells to High-Frequency Nanomechanostimulation. Biomaterials 292, 121866 (2023).
- Bizarre Effects of Sound Waves Could Benefit Drug Delivery, Exosome Therapy. Bio-IT World, 6 January 2021.
- Sonic Advance: How Sound Waves Could Help Regrow Bones. Scientific Inquirer, 28 February 2022.